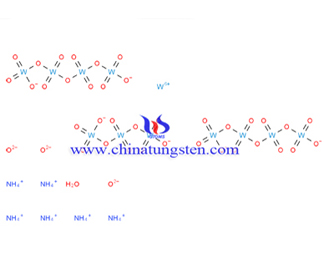
Ammonium Metatungstate Molecular Weight

Ammonium metatungstate molecular weight generally refers to relative molecular mass of ammonium metatungstate (AMT), molecular weight differences between each other depending on its moisture content; while according the formula of (NH4)6H2W12O40.XH2O, the anhydrous basis of molecular weight should be 2956.08.
Wherein the relative molecular mass of each element is:N=14, H=1, W=183.84, O=16;
then Mr(AMT)=14*6+1*26+183.84*12+16*40=2956.08.
Sum of relative atomic mass (Ar) of each element in the formula is the relative molecular mass, which represented by symbol of Mr. Relative molecular mass equal to the molar mass in value, but in different units. The unit of relative molecular mass is "1", while the unit of molar mass is g/mol. H2O is the oxide with the minimum relative molecular mass, however, as for the polymer, its relative molecular mass can up to tens or even hundreds of thousands.